Medical Device R&D
Imaging Device for Lumbar Puncture Site Identification
This startup was developing a handheld device to aid in spinal punctures. Their device integrates imaging of the spine with needle guidance to help physicians correctly insert a variety of procedure needles on the first try.
They initially requested support with an ergonomics evaluation and creation of Human Factors documents in preparation for a Pre-Submission meeting with the FDA. Documents included a Task Analysis, UFMEA, HF Plan, and a Formative Testing Protocol. Following the completion of these documents, the scope was increased to include Formative Testing support, Industrial Design management, instruction design (QRG and IFU), user interface/experience design, packaging consultation, and regulatory guidance throughout.
HF Document Creation
Kick-off meeting and design review to understand device and progress to-date
Task Analysis to inform the UFMEA
UFMEA to inform the research protocol
Protocol to guide Formative Testing
Formative Testing to begin to validate risk hypotheses

Industrial Design
Ergonomics evaluation and Industrial Design overhaul to incorporate ergonomics recommendations

UI/UX Design
Workflow mapping, user needs mapping, information architecture, and visual design to create the embedded UI

Instructional Design
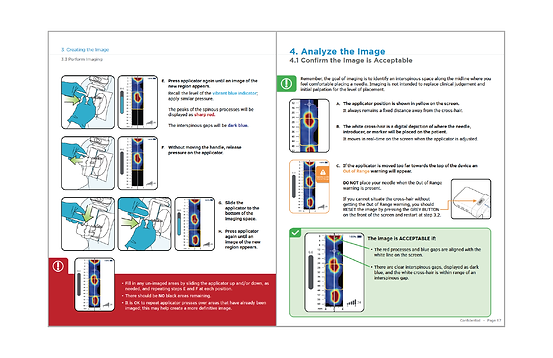
Collaboration with a medical illustrator to design and develop the device Instructions for Use (IFU) and Quick Reference Guide (QRG)